- Have any questions?
- 085913 90567
- drgeorgechestdiseases@gmail.com
The Role of Biomarkers in Lung Cancer: From Detection to Personalized Therapy

10 Harmful Effects of Cigarette Smoking
October 16, 2025
Myths of Robotic Thoracic Surgery: Debunking Common Misconceptions
October 17, 2025Lung cancer remains one of the most challenging cancers worldwide, largely because of its complexity and the difficulty in detecting it early. Even when imaging or symptoms prompt suspicion, definitive diagnosis often depends on invasive biopsies and histopathology. In many regions, delayed diagnosis is common, and only when the disease is advanced do patients seek medical attention. To improve detection and tailor therapy, researchers have turned to molecular tools — notably, the concept of a biomarker for lung cancer.
“In our ongoing research at Tata Memorial, we are refining liquid biopsy methods to detect EGFR mutations noninvasively, aiming to make biomarker testing for lung cancer more accessible,” says Dr. George Karimundackal in his recent publication.
Dr. George Karimundackal is a renowned thoracic surgeon in Mumbai and a molecular researcher with deep expertise in lung cancer biomarkers, especially liquid biopsy techniques. He has led collaborative work on noninvasive mutation analysis from circulating tumor DNA (ctDNA) in lung cancer. His work strengthens the bridge between surgical oncology and precision medicine, with a focus on biomarker testing for non small cell lung cancer and other lung biomarkers.
So, first, let’s see what exactly a biomarker is and why it matters in cancer diagnosis.
What Are Biomarkers?
Biomarkers are measurable indicators — often molecules like DNA, RNA, proteins — that reflect some biological state or disease process. In cancer, a biomarker might signal the presence of malignant cells or a mutation driving tumor growth.
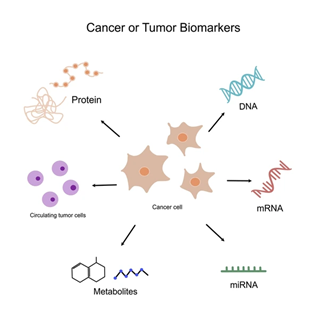
- They can be derived from tissue, blood (liquid biopsy), or other fluids.
- They may be diagnostic (indicating disease), prognostic (indicating likely course), or predictive (indicating response to therapy).
How are biomarkers used to detect lung cancer? Let’s uncover their role in early detection and tracking.
How Do Biomarkers Help in Lung Cancer Detection?
Biomarkers elevate lung cancer detection beyond imaging or routine pathology in several ways:
Noninvasive sampling via liquid biopsy
Circulating tumor DNA (ctDNA) — fragments of tumor-derived DNA released into the bloodstream — can be captured through a simple blood draw. This allows biomarker testing for lung cancer in cases where tissue biopsy is inaccessible or risky.
Complementing tissue biopsy
Tumors are heterogeneous, and a single biopsy may miss mutations. Biomarker testing can detect additional clones or evolving mutations missed by tissue sampling.
Early detection and monitoring
Rising levels of a biomarker may precede radiographic progression, enabling earlier intervention or therapy adjustments.
Risk stratification and screening (in future)
While still under development, certain biomarkers may eventually help screen high-risk populations (e.g. smokers) before tumors become visible on imaging.
Which biomarkers are key in lung cancer diagnosis? Let’s dive into the most important ones to know.
Key Biomarkers for Lung Cancer Diagnosis and Prognosis
In lung cancer — particularly non small cell lung cancer biomarkers — several molecular drivers have clinical relevance. Below are some of the most widely studied:
EGFR biomarker / EGFR biomarker lung cancer
Epidermal growth factor receptor (EGFR) mutations, especially exon 19 deletions and exon 21 L858R, are central in lung adenocarcinoma. Detection of EGFR mutations in ctDNA has shown high concordance with tumor tissue. For example, a study using BEAMing PCR showed concordance rates over 95% for major EGFR exons.
ALK, ROS1, RET, MET, BRAF, KRAS
These gene rearrangements or mutations are commonly evaluated in biomarker testing for non small cell lung cancer per NCCN and other guidelines.
PD L1 expression & Tumor Mutational Burden (TMB)
Though not a genetic marker, PD‑L1 and TMB are used to predict immunotherapy response in NSCLC.
ctDNA signatures / liquid biopsy panels
Beyond EGFR, ctDNA panels (using methods like CAPP‑Seq) can detect multiple lung biomarkers, even at very low levels.
Proteomic signatures (e.g. VeriStrat)
Tests like VeriStrat categorize patients into “good” or “poor” prognostic groups and may predict response to EGFR inhibitors.
Small cell lung cancer biomarkers / lung adenocarcinoma biomarkers
In small cell lung cancer (SCLC), biomarkers such as DLL3, neuroendocrine markers (e.g. synaptophysin, chromogranin), and circulating tumor cells (CTCs) are studied but clinical use remains limited.
Knowing which biomarkers matter can change the course of treatment. Speak with a professional to see which tests may be relevant.
Contact NowWhich biomarkers are key in lung cancer diagnosis? Let’s dive into the most important ones to know.
How Biomarkers Influence Treatment Decisions
Biomarkers are not just for detection—they actively shape therapy choices in lung cancer.
Targeted therapy selection
If a patient has an actionable EGFR mutation, EGFR tyrosine kinase inhibitors (TKIs) like osimertinib can be used. If ALK or ROS1 fusions are found, corresponding inhibitors are chosen instead.
Resistance monitoring and adaptation
Following therapy, biomarkers can track emergent resistance mutations (e.g. EGFR T790M or C797S). Detection in ctDNA helps decide when to switch treatments.
Immunotherapy suitability
High PD‑L1 or high TMB may indicate likely benefit from immune checkpoint inhibitors.
Minimal residual disease (MRD) detection
Post-surgery or therapy, very low levels of ctDNA may predict relapse before imaging picks it up.
De-escalation or escalation
If biomarker levels fall dramatically, therapy might be reduced; if they rise, more aggressive therapy or combination regimens may be needed.
What’s coming next in lung cancer biomarker research? Let’s explore the future breakthroughs ahead.
The Future of Biomarkers in Lung Cancer Care

The landscape is evolving rapidly. Here are promising trends:
Ultra sensitive sequencing and error correction
Techniques (e.g. CAPP‑Seq, iDES) can detect one mutant DNA molecule among thousands of normal ones.
Artificial intelligence and computational biomarkers
AI models combining histology images and molecular data are being developed to predict EGFR status and resistance. For example, an H&E-based computational biomarker achieved AUC ~84% in EGFR prediction.
Multimodal prediction models
Integrating clinical data, imaging, genomics, and pathology to forecast resistance and guide therapy in NSCLC.
Expanded panels and pan cancer liquid biopsy
Broader ctDNA panels covering dozens of genes could identify rare actionable mutations even before tumor detection.
Point-of-care biomarker assays
As costs fall, rapid assays may become feasible in outpatient clinics, especially in resource-limited settings.
Integration into screening programs
In future, biomarker panels might augment low-dose CT or be used as preliminary screens for high-risk individuals.
What do biomarkers mean for patients? Let’s discover the real-world benefits they offer.
Benefits of Biomarkers for Lung Cancer Patients

Here’s what patients stand to gain:
- Less reliance on repeated invasive biopsies
- Faster, more precise diagnosis and treatment start
- Tailored therapy, reducing unnecessary side effects
- Early detection of recurrence or resistance
- Improved overall patient monitoring and adaptability
Biomarker testing for lung cancer is shifting the paradigm — from “one-size-fits-all” to personalized care.
Conclusion
Using a biomarker for lung cancer has transformed how we detect, prognosticate, and treat lung cancer. What once required large tissue samples and guesswork can now often be achieved through a simple blood draw. Especially in non small cell lung cancer, biomarkers like EGFR, ALK, ROS1, and ctDNA signatures drive targeted therapy decisions and enable real-time monitoring. With advances in sequencing, AI, and multimodal integration, the future of lung cancer care is increasingly data-driven and patient-centric.
As Dr. George Karimundackal continues his work bridging surgery and molecular diagnostics, his research promises to expand access to biomarker testing and refine strategies in lung cancer — delivering better outcomes for patients everywhere.
Every patient deserves tailored care backed by science. Reach out to a specialist to discover how biomarkers may benefit you.
Contact NowFAQs
The most common biomarkers include EGFR mutations (especially exon 19 deletions or exon 21 L858R), ALK and ROS1 rearrangements, BRAF, MET exon 14 skipping, and PD‑L1 expression.
They enable tailored therapies (e.g. targeted drugs), allow early detection of resistance, and help monitor treatment response — all leading to more effective, less toxic treatment.
Costs vary significantly by country, lab, and panel (single gene vs multigene), but can range from tens to hundreds or even thousands of dollars (or equivalent currency). Insurance or government programs may cover part of the cost.
Yes — predictive biomarkers like EGFR status or PD‑L1 can indicate a likely response to certain targeted therapies or immunotherapy.
Yes — especially liquid biopsy (blood test), which is minimally invasive and carries a very low risk.
Disclaimer: The information shared in this content is for educational purposes and not for promotional use.

